
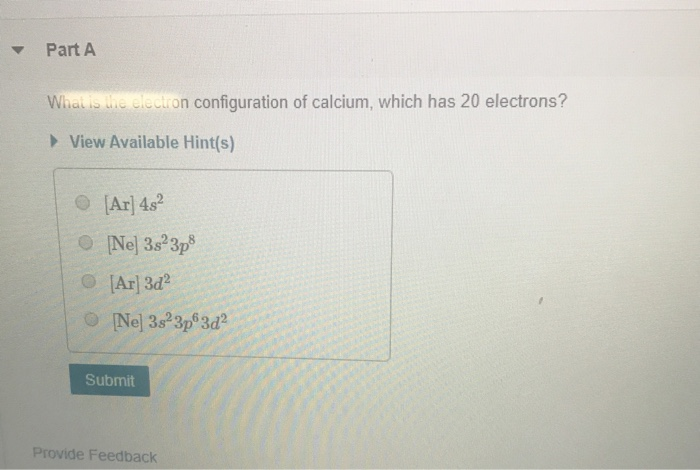
Electronic configuration of Co +2 is 3d 7. You will generally be on the move quite a lot anyway, considering the first service interval of the BMW CE 04 is a whole 24 months or 6,214 miles. In this video, Arvind sir will cover Question 8. The 4f electrons of Gd are treated as core states because the Gd 4f … Electron configuration describes the distribution of electrons within an atom. View solution > The outer shell electronic configuration of G d (Z = 6 4) is. 2 is useful in remembering the order for electrons to occupy shells and subshells. The calcium ion (Ca 2+), however, has two electrons less. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In which of the following sets do all species have the same number of protons? D) O, O2-, O2+.
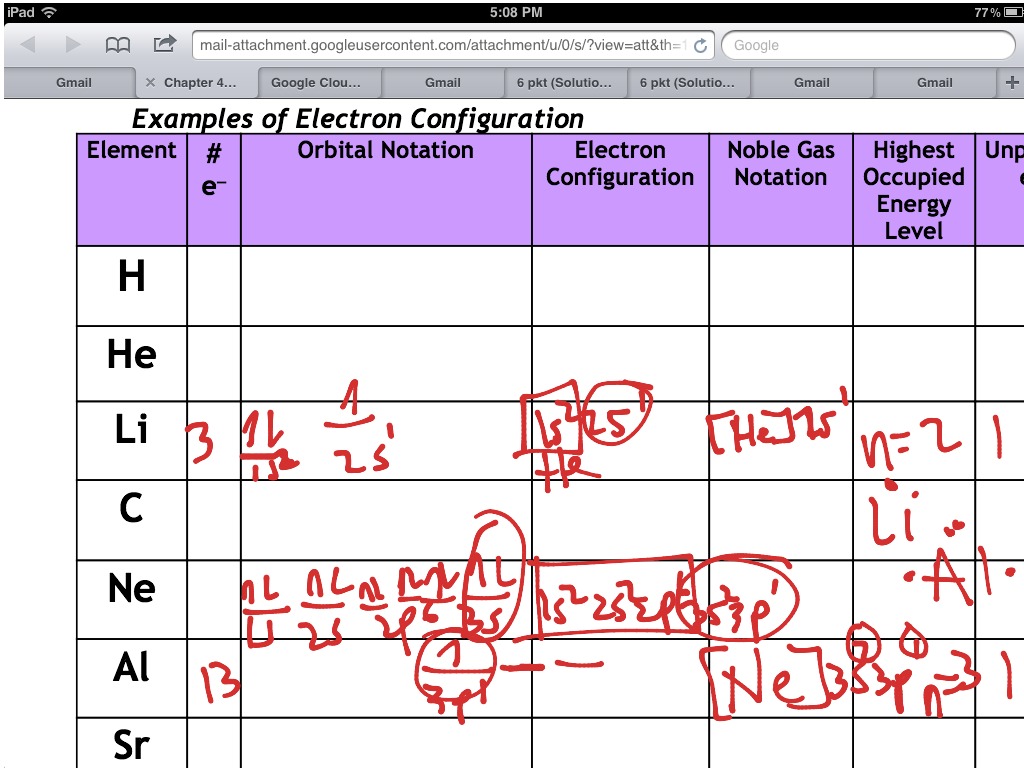
This electron is located on the second energy level, in the 2s-orbital. The electronic configuration is as follows: 1s2 2s2 2p6 3s2 3p6 4s1. Their electronegative value is nearly equal to s-block elements. 1 Study App and Learning App with Instant Video Solutions for NCERT Class 6, Class 7, Class 8, Class 9, Class 10, Class 11 and Class 12, IIT JEE prep, NEET preparation and CBSE, UP Board, Bihar Board, Rajasthan Board, … Write the general electronic configuration of lanthanoids. A good place to start when trying to figure out the electron configuration of an ion is the electron configuration of the neutral parent atom. Promethium is an element with atomic number 61 and thus contain 61 electrons. However this same transition should result in intense visible emission from C e 3 +, and to the best of our knowledge, this has not been reported in any tellurite glass.
Electron configuration of Cerium is 4f1 5d1 6s2. And it has 19 electrons that will be assigned to the s and p subshells. Whereas in Sm +2, the lanthanides are more stable in +3 state and hence in +2 state it gets oxidized easily (good reducing agent) to get Key People: electronic configuration, also called electronic structure or electron configuration, the arrangement of electrons in orbitals around an atomic nucleus. The electron configuration is: 4f15d16s2 4 f 1 5 d 1 6 s 2 Since the d and f electrons are in different subshelles, they are non-equivalent electrons and … Ce 4+: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 Or, 54. KMnO 4 is not acidified by HCl instead of H 2 SO 4 because. Which among the following statement is wrong (1) Electronic configuration of Gd, is 4f75d4682 (2) Ce4+ is a good reductant (3) Actinoids exhibit … Cerium is a chemical element with the symbol Ce and atomic number 58. Therefore, it forms C e 4 + ion in aqueous solution. Its outermost orbit contains 2 electrons so Eu²⁺ is stable. cerium (ce) and ytterbium (yb) are both group 3b metals that exhibit other oxidation states in addition to the expected 3 state. Ions with + 4 oxidation state act as oxidising and ions having The Ce metal has the following electronic configuration: 4f✕d✖s². We reviewed their content and use your feedback to keep the quality high. Those tiny little subatomic particles, whizzing around in their orbits.Electronic configuration of ce4. Saturated Unsaturated and SupersaturatedĪh, electrons.Reaction Quotient and Le Chatelier's Principle.Prediction of Element Properties Based on Periodic Trends.Molecular Structures of Acids and Bases.

Ion and Atom Photoelectron Spectroscopy.



 0 kommentar(er)
0 kommentar(er)
